The Brick uses FCS and smFRET to explore molecules one at a time, capturing real-time molecular behavior, interactions, and structural changes at the single-molecule level.
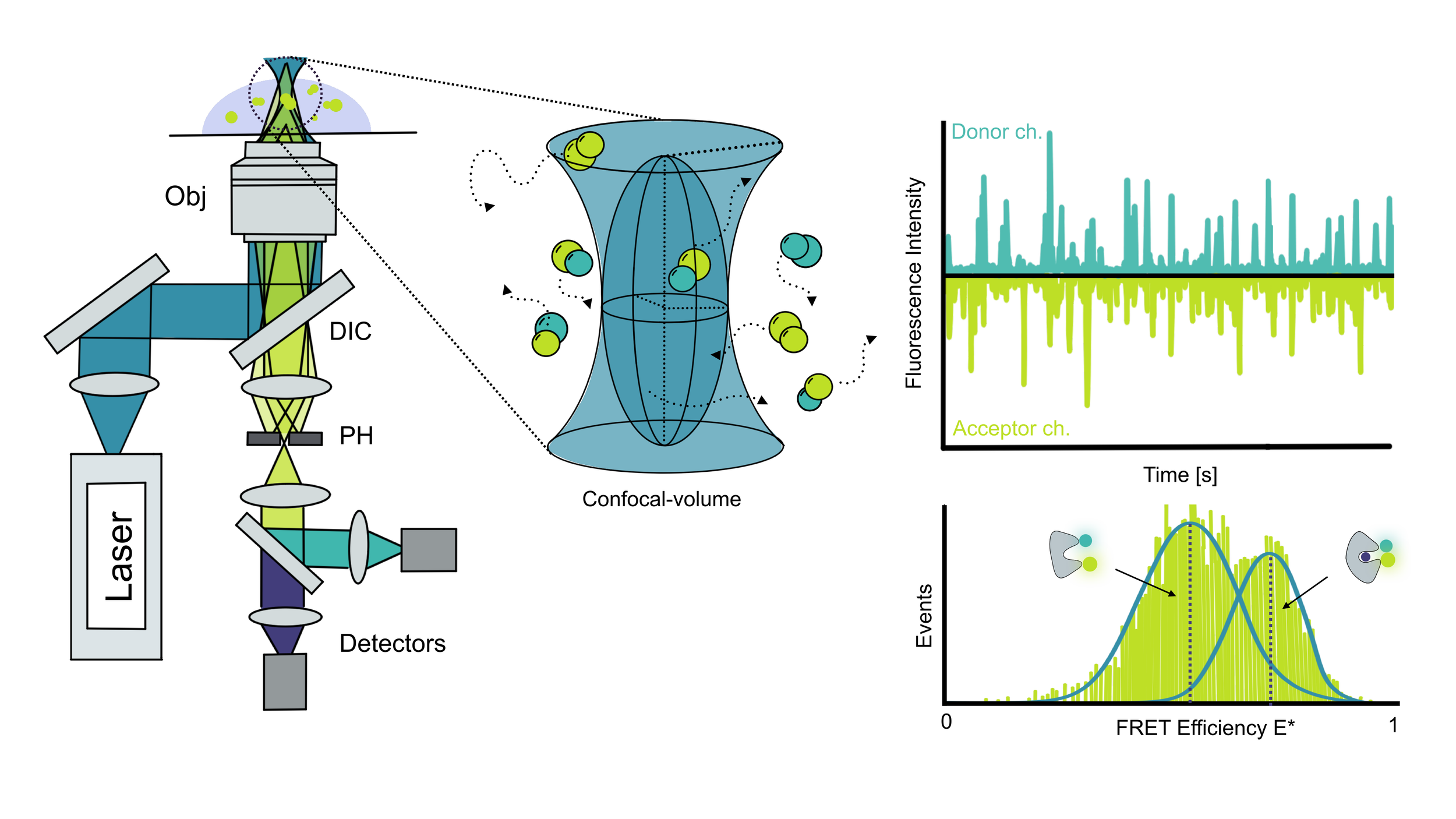
Fluorescence Correlation Spectroscopy (FCS) measures fluctuations in fluorescence from a fluorescent protein or a biomolecule labeled with an organic dye as the molecules move through the focus, also called the confocal volume. As molecules diffuse randomly in and out of this tiny detection spot, FCS records the resulting fluorescence intensity fluctuations over time.
FCS is highly flexible, the only requirement is that your biomolecule, particle, or system of interest fluoresces. From there, a wide range of applications opens up:
Protein–protein interactions: Detect binding events by observing changes in diffusion times.
Aggregation and fibril formation: Monitor how molecules assemble over time, from early oligomers to larger structures.
By autocorrelating the recorded time traces and fitting them with appropriate models, FCS provides quantitative information about diffusion dynamics, molecular concentration, and interactions at the single-molecule level.
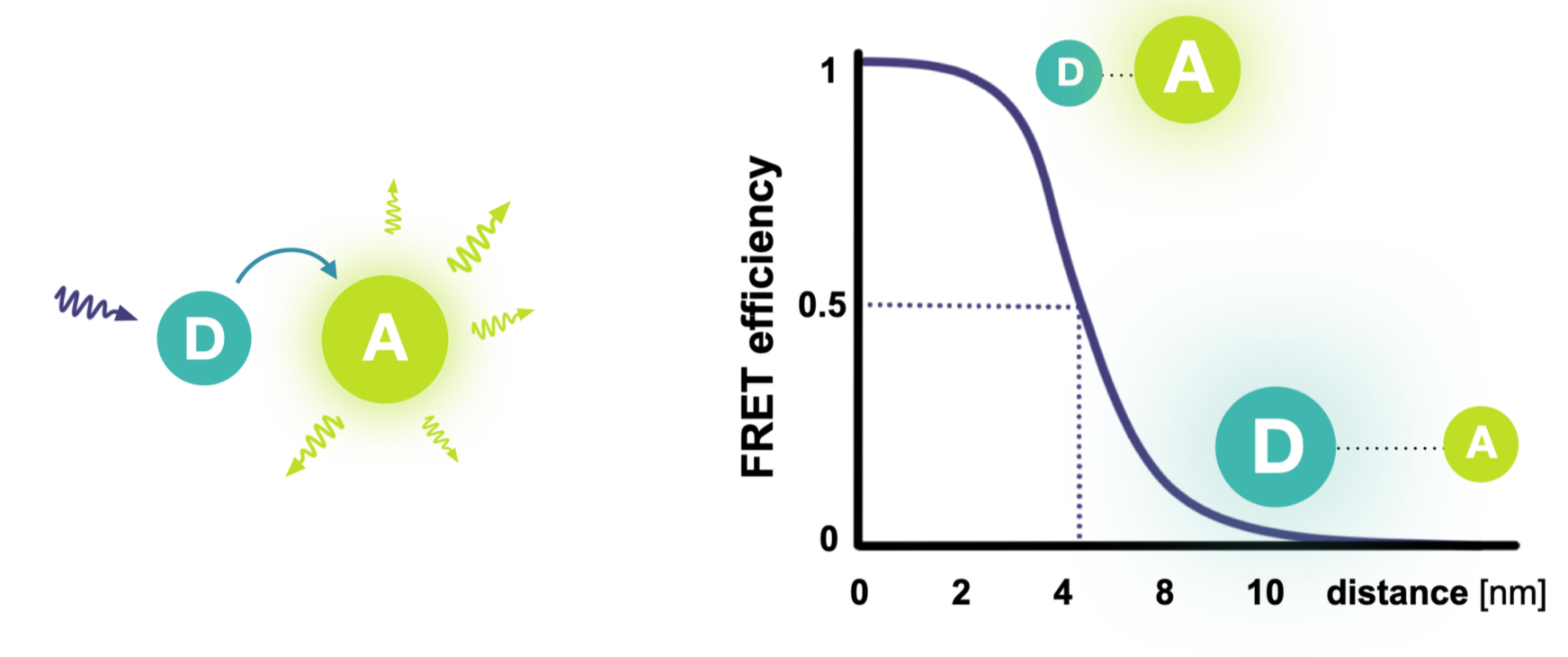
Förster energy transfer describes how an excited donor dye (D) can pass its energy to a nearby acceptor dye (A), making it fluoresce.
Because the transfer efficiency depends strongly on the distance between the dyes, smFRET acts as a precise nanometer-scale ruler.
By attaching the dyes to a protein, smFRET can directly reveal conformational changes and molecular interactions.
In contrast to FCS, smFRET is a pure single-molecule technique. Molecules diffuse in and out of the focus, but the sample is diluted so that, on average, only one molecule enters the detection volume at a time, producing a burst of fluorescence in both donor and acceptor channels.
This setup makes it possible to separate donor-only and acceptor-only species from properly labeled FRET samples containing both dyes.
Each fluorescence burst is then analyzed individually. From the intensity in the two channels, the FRET efficiency (energy transfer between donor and acceptor) can be calculated. Collecting many bursts builds a histogram of FRET efficiencies, which is then fitted (e.g., with a Gaussian) to extract quantitative information about molecular conformations and dynamics.
For FCS
Only a fluorescence signal is needed. Biomolecules tagged with GFP are ready to use, or they can be labeled unspecifically with an organic dye:
NHS chemistry targets lysines.
Maleimide chemistry targets cysteines.
The key is to ensure that the targeted amino acids are not part of functional regions, such as binding pockets.
For smFRET
Labeling requires more preparation. smFRET relies on site-specific labeling, most often achieved by site-directed mutagenesis to introduce cysteines at defined positions. Good labeling sites should meet three criteria:
Not located in conserved regions.
Surface-exposed for accessibility.
Provide a good dynamic range, so the donor–acceptor distance changes between conformational states.
To support experimental design, the Cordes Lab developed theLabelizer, a tool that analyzes protein structures (PDB files) and suggests optimal labeling positions for smFRET.